·经验与总结·
无症状人群中体检筛查的循证医学证据
周江华1,杨 颖1,邹 川1,董碧蓉2
(1.成都市第五人民医院老年科,四川 成都 611130; 2. 华西医院老年医学中心,四川 成都 610041)
【摘要】目的:检索全球体检项目证据,开发基于循证医学证据的体检项目软件。方法:计算机检索美国国立指南库(NGC)、Cochrane library(2017年第3期)、PubMed、Embase、CBM、CNKI、VIP和WanFang Data等数据库。搜集所有健康体检项目的高质量证据。检索时限均为2005年1月至2017年3月。由2名研究者独立筛选文献、提取资料。结果:共纳入183篇文献,包括23篇高质量的RCT、82篇指南和78篇系统评价。基于现有证据,27项体检项目在成人中进行筛查有临床意义,5项体检项目进行筛查无临床意义(尿酸、CEA、CA19-9、PSA、骨盆检查)。推荐当前体检项目增加腹主动脉超声、乳腺钼靶、结肠镜、黑色素瘤和老年综合评估进行筛查。结论:当前体检模式尚存在缺陷,基于循证医学证据的体检筛查模式迫切需要。
【关键词】循证医学;体检;筛查
我国面临着慢性疾病巨大挑战。2009年我国高血压疾病患者已超过1.6亿,冠心病新发病例以每年75万的速度递增。60岁以上老年人中,有2/3的时间处于带病生存状态,部分失能和全失能老人已超过3650万。研究预测到2030年,因老年人健康问题会抵消我国年人均GDP 7%的增幅。因此,医疗服务的根本出路在于“预防疾病”,早检查、早预防、早发现、早治疗,这是解决“看病难、看病贵”,降低医疗费用的有效途径之一。
目前很多健康体检机构提供“套餐”式服务,但内容往往是根据价格不同来制定的,价格越高,体检的项目越多。然而,很多因素在健康体检中有着举足轻重的意义,如年龄、居住环境、职业等。另外,老年人也因功能状态、衰弱程度、预期寿命的不同,疾病筛查重点应该与非老年成年人不同,不能简单地沿用成人的疾病筛查方法。韩国、日本因胃癌发生率高,尤其分别强调在40~75岁[1]和50岁以上成年人[2]中使用胃镜进行筛查。因此,建立成人健康体检和疾病筛查方法的循证医学证据库,对疾病筛查的利弊进行分析和结果给予科学解释,并提出适应临床的推荐意见尤为迫切和重要。本研究旨在查询各项体检项目的体检证据,以助于建立一套基于循证医学证据的体检模式。
1 资料与方法
1.1 纳入与排除标准
1.1.1 研究类型 关于体检项目筛查的临床指南、专家共识、系统评价、Meta分析、大型随机对照研究。文种限中文、英文。
1.1.2 研究对象 无症状的成人。
1.1.3 暴露因素 采用在无症状人群中进行体检筛查。体检筛查是以无症状人群为体检对象,以疾病预防为目的,在医疗单位或体检机构进行的身体检查。具体体检项目参考成都市三甲医院体检中心开展的体检项目、《四川省干部体检项目推进内容》、《卫生部健康体检项目目录》、2014年《健康体检基本项目专家共识》[3],体检项目如表1。
表1健康体检项目目录

项目类别主要检查内容必选项目 一般检查身高,体质量,体质量指数,腰围,血压,脉搏 物理检查内科,外科,眼科,耳鼻喉科,口腔科,女性妇科 实验室检查血常规,尿常规,尿微量白蛋白与肌酐比值,大便隐血;肝功能(总蛋白,白蛋白,球蛋白,谷丙转氨酶,谷草转氨酶,乳酸脱氢酶,γ-谷氨酰转肽酶,碱性磷酸酶,总胆红素,直接胆红素,间接胆红素);肾功能(尿素氮,肌酐,尿酸);血脂(总胆固醇,三酰甘油,低密度脂蛋白胆固醇,高密度脂蛋白胆固醇);妇科病理学检查;空腹血糖 辅助检查心电图,腹部超声(肝,胆,胰,脾,肾)备选检查 实验室检查甲状腺功能测定,糖化血红蛋白,肿瘤标志物(癌胚抗原,甲胎蛋白,血清CA19-9抗原,女性血清CA125抗原,男性前列腺特异抗原),HPV 辅助检查甲状腺彩超,女性乳腺彩超,胸部CT,心脏彩超,颈动脉彩超,骨密度
1.1.4 结局指标 死亡率、敏感性、特异性、假阳性率。
1.1.5 排除标准 (1)会议摘要;(2)研究人群为儿童或者孕妇;(3)重复发表的文献;(4)同一试验前期发表的研究。
1.2 文献检索策略 计算机检索数据库Uptodate、美国国立指南库(NGC)、美国预防服务工作组、加拿大周期性检查服务工作组、美国医学会、世界卫生组织、美国胸外科协会、美国疾病与预防控制中心、美国癌症协会、美国糖尿病协会、美国甲状腺协会、美国国家综合癌症网络指南,英国国家临床研究所、Cochrane library、PubMed、Embase、CBM、CNKI、VIP和WanFang Data等数据库,搜集所有健康体检项目的高质量证据。检索时限均为2005年1月至2017年3月。英文检索词包括 screen、screening、systematic review、meta-analysis、randomized controlled trial、guideline。中文检索词包括:普查、筛查、随机对照、系统评价、Meta分析。
1.3 文献筛选与资料提取 由2位研究者独立进行文献筛选和资料提取,并交叉核对,若遇分歧则通过讨论解决或交由第三方协助裁定。采用自制的资料提取表提取资料,提取内容主要包括:第一作者、发表年限、调查地区、样本量大小、年龄、研究时间段、研究类型、随访时间和主要结局等。
1.4 纳入研究质量评价 本研究主要纳入的研究类型为指南、系统综述和随机对照研究,未进行研究的质量评价。
1.5 结果分析 本研究根据Uptodate、美国国立指南库(NGC)、美国预防服务工作组、加拿大周期性检查服务工作组、美国医学会、世界卫生组织、美国胸外科协会等指南结果对筛查项目做出最终筛查意见。当指南筛查意见不一致时,本研究采用Uptodate的建议为本研究结论。
2 结 果
2.1 文献检索结果 初检出相关文献19 336篇,经逐层筛选后,最终纳入183篇英文文献。
2.2 纳入研究基本信息 本文共纳入23篇高质量的RCT[4-26]、82篇指南和78篇系统评价(见表2)。
表2体检项目相关的二次研究和指南
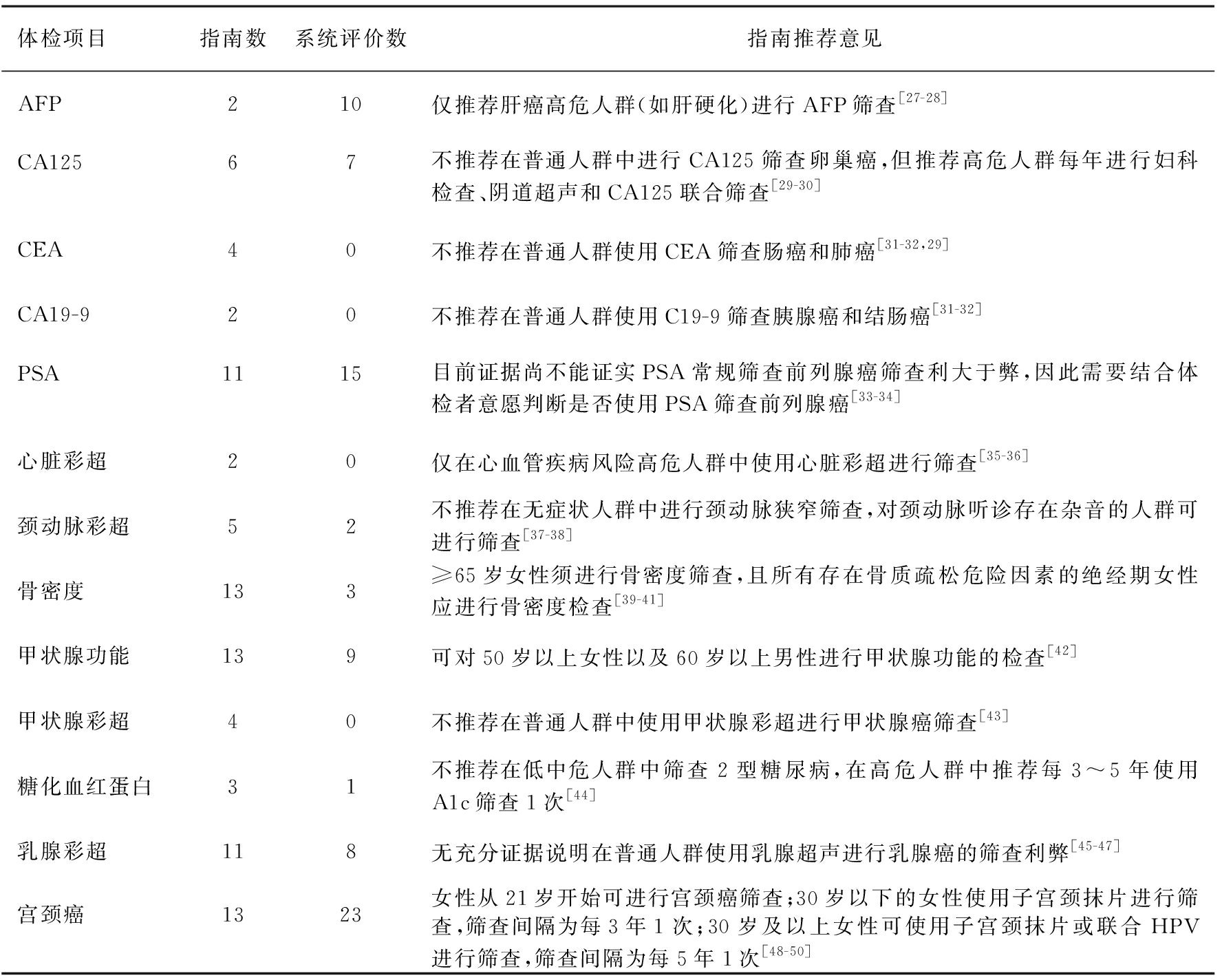
体检项目指南数系统评价数指南推荐意见AFP210仅推荐肝癌高危人群(如肝硬化)进行AFP筛查[27-28]CA12567不推荐在普通人群中进行CA125筛查卵巢癌,但推荐高危人群每年进行妇科检查、阴道超声和CA125联合筛查[29-30]CEA40不推荐在普通人群使用CEA筛查肠癌和肺癌[31-32,29]CA19-920不推荐在普通人群使用C19-9筛查胰腺癌和结肠癌[31-32]PSA1115目前证据尚不能证实PSA常规筛查前列腺癌筛查利大于弊,因此需要结合体检者意愿判断是否使用PSA筛查前列腺癌[33-34]心脏彩超20仅在心血管疾病风险高危人群中使用心脏彩超进行筛查[35-36]颈动脉彩超52不推荐在无症状人群中进行颈动脉狭窄筛查,对颈动脉听诊存在杂音的人群可进行筛查[37-38]骨密度133≥65岁女性须进行骨密度筛查,且所有存在骨质疏松危险因素的绝经期女性应进行骨密度检查[39-41]甲状腺功能139可对50岁以上女性以及60岁以上男性进行甲状腺功能的检查[42]甲状腺彩超40不推荐在普通人群中使用甲状腺彩超进行甲状腺癌筛查[43]糖化血红蛋白31不推荐在低中危人群中筛查2型糖尿病,在高危人群中推荐每3~5年使用A1c筛查1次[44]乳腺彩超118无充分证据说明在普通人群使用乳腺超声进行乳腺癌的筛查利弊[45-47]宫颈癌1323女性从21岁开始可进行宫颈癌筛查;30岁以下的女性使用子宫颈抹片进行筛查,筛查间隔为每3年1次;30岁及以上女性可使用子宫颈抹片或联合HPV进行筛查,筛查间隔为每5年1次[48-50]
注:指南有7篇重复,指南总数为82篇。
肿瘤标志物常用于临床诊断,但现有的证据不支持使用肿瘤标志物进行筛查[29-36],缺乏CA19-9、AFP和CEA筛查的高质量随机对照研究。2个欧洲随机对照研究推荐瘤体分别3~5.4 cm、<5 cm腹主动脉瘤中,定期使用超声筛查[10-11]。然而,不推荐使用彩超筛查心血管疾病、颈动脉狭窄和甲状腺癌[37-40,45]。乳腺癌和宫颈癌是最常见的妇科肿瘤。美国癌症协会和美国妇产科协会推荐40岁以上的女性每年进行1次钼靶筛查[48];美国预防工作组推荐在 21岁开始可进行宫颈癌筛查,30岁以下的女性使用子宫颈抹片进行筛查,30岁以上的女性使用子宫颈抹片或联合HPV进行筛查[51,50]。
2.3 体检证据汇总 健康体检项目共36项,包括23项必选体检项目和13项备选项目。其中27项体检项目有证据支持在成人中进行筛查,5项体检项目无证据支持筛查有临床意义,4项体检项目未找到高质量的临床证据。根据当前的体检项目,现有证据推荐增补腹主动脉超声、乳腺钼靶、结肠镜、黑色素瘤、老年综合评估等体检项目。详见表3。
表3体检项目筛查证据一览表

目 录具体项目基于循证医学证据可进行筛查的体检项目体质量指数、腰围、外科、眼科、耳鼻喉科、口腔科、尿常规、大便隐血、尿微量白蛋白与肌酐比值、尿素氮、肌酐、肝功能、血脂[51]、空腹血糖、糖化血红蛋白、甲状腺功能测定、AFP[52]、CA125、宫颈液基细胞学、丙肝检测[53]、甲状腺超声、乳腺彩超、胸部CT、静息心电图、眼压测定、心脏彩超、颈动脉彩超[54]、骨密度、女性妇科常规基于循证医学证据不必进行筛查的体检项目尿酸、CEA、CA19-9、PSA、骨盆检查[55]基于循证医学证据需要补充的体检项目腹主动脉超声[56]、乳腺钼靶、结肠镜[57]、黑色素瘤[58]、老年综合评估[59]尚无证据说明的体检项目内科、血常规、腹部超声、血沉
3 讨 论
3.1 健康体检项目的临床实际意义 绝大多数体检项目在成年人中进行常规筛查有临床证据支持,但证据之间存在矛盾。2011年发表的美国国立肺筛查试验(the National Lung Screening Trail)研究证实,在长期吸烟的55~74岁高风险人群中,通过每年1次胸部低剂量CT(LDCT)筛查,可使肺癌死亡率降低20%。在进一步检索和评估后,美国预防服务工作组推荐对55~80岁的高风险人群进行筛查,但如果戒烟超过15年,或有其他影响寿命的疾病,则不建议持续筛查[60]。美国癌症协会推荐40岁以上的女性每年进行1次钼靶筛查,但没有明确筛查的终止年龄。然而美国妇产科协会推荐75岁以上老年人应咨询医生,决定是否应该进行钼靶筛查[61,46]。
某些项目在体检机构中过度使用或被忽略。CEA、CA19-9等体检项目可降低减少死亡率,但其敏感性低、假阳性率高可引起过度诊断,影响体检者的心理状态和生活质量[9-12]。腹主动脉瘤是最常见的动脉瘤,其特征为无明显临床症状,易发生瘤体破裂,死亡率极高。使用超声筛查能够实现早期诊断,降低瘤体破裂导致死亡的风险[56]。
3.2 体检模式的优缺点 当前体检模式往往是“套餐”式,其可筛查部分疾病,但其假阳性率高,并极大影响体检者心理,造成医疗资源过度浪费。冯雨来等[62]研究强调将价格套餐模式向风险套餐模式转变,但基于危险因素做出体检项目的推荐很有可能导致某些重要体检项目被忽略。因此,基于循证医学证据的体检筛查模式迫切需要。
3.3 本研究缺点和局限 本研究仅纳入已经发表的文献,缺乏灰色文献。本研究未对纳入研究文献进行质量评价。
综上所述,当前体检模式尚存在缺陷,迫切需要建立基于循证医学证据的体检筛查模式。
参考文献:
[1]LEE J H, KIM J G, JUNG H K, et al. Synopsis on clinical practice guideline of gastric cancer in Korea: an evidence-based approach[J]. Korean J Gastroenterol, 2014, 63(2): 66-81.
[2]HAMASHIMA C, SHIBUYA D, YAMAZAKI H, et al. The Japanese guidelines for gastric cancer screening[J]. Jpn J Clin Oncol, 2008, 38(4): 259-267.
[3]中华医学会健康管理学分会.中华健康管理学杂志编委会.健康体检基本项目专家共识[J].中华健康管理学杂志,2014,8(2):81-90.
[4]SCHRÖDER F H, HUGOSSON J, ROOBOL M J, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up[J]. Lancet, 2014, 384(9959): 2027-2035.
[5]ANDRIOLE G L, CRAWFORD E D, GRUBB R L, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up[J]. J Natl Cancer Inst, 2012, 104(2): 125-132.
[6]KJELLMAN A, AKRE O, NORMING U, et al. 15-year followup of a population based prostate cancer screening study[J]. J Urol, 2009, 181(4): 1615-1621; discussion 1621.
[7]PARTRIDGE E, KREIMER A R, GREENLEE R T, et al. Results from four rounds of ovarian cancer screening in a randomized trial[J]. Obstet Gynecol, 2009, 113(4): 775-782.
[8]JACOBS I J, MENON U, RYAN A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial[J]. Lancet, 2016, 387(122): 945-956.
[9]THOMPSON S G, ASHTON H A, GAO L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening[J]. British Journal of Surgery, 2012, 99(12): 1649-1655.
[10]LINDHOLT J S, JUUL S, FASTING H, et al. Screening for abdominal aortic aneurysms: single centre randomised controlled trial[J]. BMJ, 2005, 330(7494): 750.
[11]LINDEKLEIV H, LØCHEN M L, MATHIESEN E B, et al. Echocardiographic screening of the general population and long-term survival: a randomized clinical study[J]. JAMA Intern Med, 2013, 173(17): 1592-1598.
[12]BARR R J, STEWART A, TORGERSON D J, et al. Population screening for osteoporosis risk: a randomised control trial of medication use and fracture risk[J]. Osteoporos Int, 2010, 21(4): 561-568.
[13]RONCO G, GIORGI-ROSSI P, CAROZZI F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial[J]. Lancet Oncol, 2010, 11(3): 249-257.
[14]RONCO G, DILLNER J, ELFSTRÖM K M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials[J]. Lancet, 2014, 383(9916): 524-532.
[15]KITCHENER H C, ALMONTE M, THOMSON C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial[J]. Lancet Oncol, 2009, 10(7): 672-682.
[16]RIJKAART D C, BERKHOF J, ROZENDAAL L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial[J]. Lancet Oncol, 2012, 13(1): 78-88.
[17]MACCALLINI V, ANGELONI C, CARACENI D, et al. Comparison of the conventional cervical smear and liquid-based cytology: results of a controlled, prospective study in the Abruzzo Region of Italy[J]. Acta Cytol, 2008, 52(5): 568-574.
[18]SULTANA F, ENGLISH D R, SIMPSON J A, et al. Home-based HPV self-sampling improves participation by never-screened and under-screened women:results from a large randomized trial(iPap)in Australia[Z], 2016: 30031.
[19]STRANDER B, ANDERSSON-ELLSTRÖM A, MILSOM I, et al. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program : a prospective randomized study[J]. Cancer, 2007, 111(5): 285-291.
[20]SIEBERS A G, KLINKHAMER P J, ARBYN M, et al. Cytologic detection of cervical abnormalities using liquid-based compared with conventional cytology: a randomized controlled trial[J]. Obstet Gynecol, 2008, 112(6): 1327-1334.
[21]NAUCLER P, RYD W, TORNBERG S, et al. Human papillomavirus and papanicolaou tests to screen for cervical cancer[J].N Engl J Med, 2007, 357(16): 1589-1597.
[22]MAYRAND M H, DUARTE-FRANCO E, RODRIGUES I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer[J].N Engl J Med, 2007, 357(16): 1579-1588.
[23]LEINONEN M K, NIEMINEN P, LONNBERG S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing:prospective randomised trial in Finland[Z], 2012: e7789.
[24]LAZCANO-PONCE E, LORINCZ A T, CRUZ-VALDEZ A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (March): a community-based randomised controlled trial[J]. Lancet, 2011, 378(986): 1868-1873.
[25]BULKMANS N W, BERKHOF J, ROZENDAAL L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial[J]. Lancet, 2007, 370(961): 1764-1772.
[26]BERG W A, BLUME J D, CORMACK J B, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer[J]. JAMA, 2008, 299(18): 2151-2163.
[27]STURGEON C M, DUFFY M J, HOFMANN B R, et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers[J]. Clin Chem, 2010, 56(6): e1-48.
[28]BRUIX J,SHERMAN M. Management of hepatocellular carcinoma: an update[J]. Hepatology, 2011, 53(3): 1020-1022.
[29]STURGEON C M, DUFFY M J, STENMAN U H, et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers[J]. Clin Chem, 2008, 54(12): e11-e79.
[30]MOYER V A. Screening for ovarian cancer:U.S.Preventive Services Task Force reaffirmation recommendation statement[J]. Ann Intern Med, 2012, 157(12): 900-904.
[31]LOCKER G Y, HAMILTON S, HARRIS J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer[J]. J Clin Oncol, 2006, 24(33): 5313-5327.
[32]DUFFY M J, VAN DALEN A, HAGLUND C, et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines[J]. Eur J Cancer, 2003, 39(6): 718-727.
[33]WOLF A M, WENDER R C, ETZIONI R B, et al. American cancer society guideline for the early detection of prostate cancer: update 2010[J]. CA Cancer J Clin, 2010, 60(2): 70-98.
[34]MOYER V A. Screening for prostate cancer:U.S.Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2012, 157(2): 120-134.
[35]GREENLAND P, ALPERT J S, BELLER G A, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines[J]. Circulation, 2010, 122(25): e584-e636.
[36]EARLS J P, WOODARD P K, ABBARA S, et al. ACR appropriateness criteria asymptomatic patient at risk for coronary artery disease[J]. J Am Coll Radiol, 2014, 11(1): 12-19.
[37]LIM L S, HAQ N, MAHMOOD S, et al. Atherosclerotic cardiovascular disease screening in adults:American College of Preventive Medicine position statement on preventive practice[J]. Am J Prev Med, 2011, 40(3): 381.e1-381.10.
[38]Summaries for patients. Screening for carotid artery stenosis: U.S. Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2014, 161(5): I-28.
[39]QASEEM A, SNOW V, SHEKELLE P, et al. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians[J]. Ann Intern Med, 2008, 148(9): 680-684.
[40]NORDIN C. Screening for osteoporosis:U.S.Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2011, 155(4): 276.
[41]LIM L S, HOEKSEMA L J, SHERIN K. Screening for osteoporosis in the adult U.S.population:ACPM position statement on preventive practice[J]. Am J Prev Med, 2009, 36(4): 366-375.
[42]LEFEVRE M L. Screening for thyroid dysfunction:U.S.Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2015, 162(9): 641-650.
[43]GHARIB H, PAPINI E, PASCHKE R, et al. American association of clinical endocrinologists, associazione medici endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules[J]. J Endocrinol Invest, 2010, 33(Suppl 5): 1-50.
[44]POTTIE K, JARAMILLO A, LEWIN G, et al. Recommendations on screening for type 2 diabetes in adults[J]. CMAJ, 2012, 184(15): 1687-1696.
[45]SIU A L. Screening for breast cancer:U.S.Preventive Services Task Force Recommendation Statement[J]. Ann Intern Med, 2016, 164(4): 279-296.
[46]SMITH R A, COKKINIDES V, BROOKS D, et al. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening[J]. CA Cancer J Clin, 2010, 60(2): 99-119.
[47]张晓光.帕丽达·帕尔哈提,张雨et al.彩色多普勒超声对女性乳腺癌诊断价值的Meta分析[J].中国循证医学杂志,2013(9):1073-1079.
[48]WILT T J, HARRIS R P, QASEEM A, et al. Screening for cancer: advice for high-value care from the American College of Physicians[J]. Ann Intern Med, 2015, 162(10): 718-725.
[49]VESCO K K, WHITLOCK E P, EDER M, et al. Risk factors and other epidemiologic considerations for cervical cancer screening:a narrative review for the U.S.Preventive Services Task Force[J]. Ann Intern Med, 2011, 155(10): 698-705.
[50]李克敏,尹如铁,康德英,等.液基细胞学对宫颈癌前病变的诊断价值:随机对照试验的系统评价[J].中国循证医学杂志,2011(10):1133-1139.
[51]HELFAND M, CARSON S S. Preventive services task force evidence syntheses,formerly systematic evidence reviews[Z], 2008.
[52]徐建业,林丁,李伟道,等.甲胎蛋白诊断原发性肝癌准确性的系统评价[J].中国循证医学杂志,2009(5):525-530.
[53]SMITH B D, MORGAN R L, BECKETT G A, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965[S], 2012: 1-32.
[54]BROTT T G, HALPERIN J L, ABBARA S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary[J]. Stroke, 2011, 42(8): e420-e463.
[55]QASEEM A, HUMPHREY L L, HARRIS R, et al. Screening pelvic examination in adult women: a clinical practice guideline from the American College of Physicians[J]. Ann Intern Med, 2014, 161(1): 67-72.
[56]LEFEVRE M L. Screening for abdominal aortic aneurysm:U.S.Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2014, 161(4): 281-290.
[57]GATES T J. Screening for cancer: concepts and controversies[J]. Am Fam Physician, 2014, 90(9): 625-631.
[58]SMITH R A, BROOKS D, COKKINIDES V, et al. Cancer screening in the United States,2013 a review of current American cancer society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening[J]. CA Cancer J Clin, 2013, 63(2): 88-105.
[59]SIU A L, US Preventive Services Task Force (USPSTF), BIBBINS-DOMINGO K, et al. Screening for depression in adults:US preventive services task force recommendation statement[J]. JAMA, 2016, 315(4): 380-387.
[60]MOYER V A. Screening for lung cancer:U.S.Preventive Services Task Force recommendation statement[J]. Ann Intern Med, 2014, 160(5): 330-338.
[61]American College of Obstetricians, BULLETIN G P. Clinical management guidelines for obstetrician gynecologists[J]. Obstet Gynecol, 2011, 118(2): 372-382.
[62]冯雨来,冯大跃,张金萍,等.国内健康体检套餐存在的问题与对策[J].中国医院,2015(3):1-2.
【中图分类号】R194.3
【文献标识码】A
DOI:10.11851/j.issn.1673-1557.2018.04.009
优先数字出版地址:http://kns.cnki.net/kcms/detail/51.1688.R.20180723.1555.058.html
通信作者:董碧蓉,birongdong@163.com
(收稿日期:2017-08-02)


